Real-World Evidence Historical Success (Pre-1962)
war-on-disease, 1-percent-treaty, medical-research, public-health, peace-dividend, decentralized-trials, dfda, dih, victory-bonds, health-economics, cost-benefit-analysis, clinical-trials, drug-development, regulatory-reform, military-spending, peace-economics, decentralized-governance, wishocracy, blockchain-governance, impact-investing
Historical Evidence: Why Real-World Evidence Works Better
Large-scale efficacy trials based on real-world evidence produce better health outcomes than current pharmaceutical industry-driven randomized controlled trials.
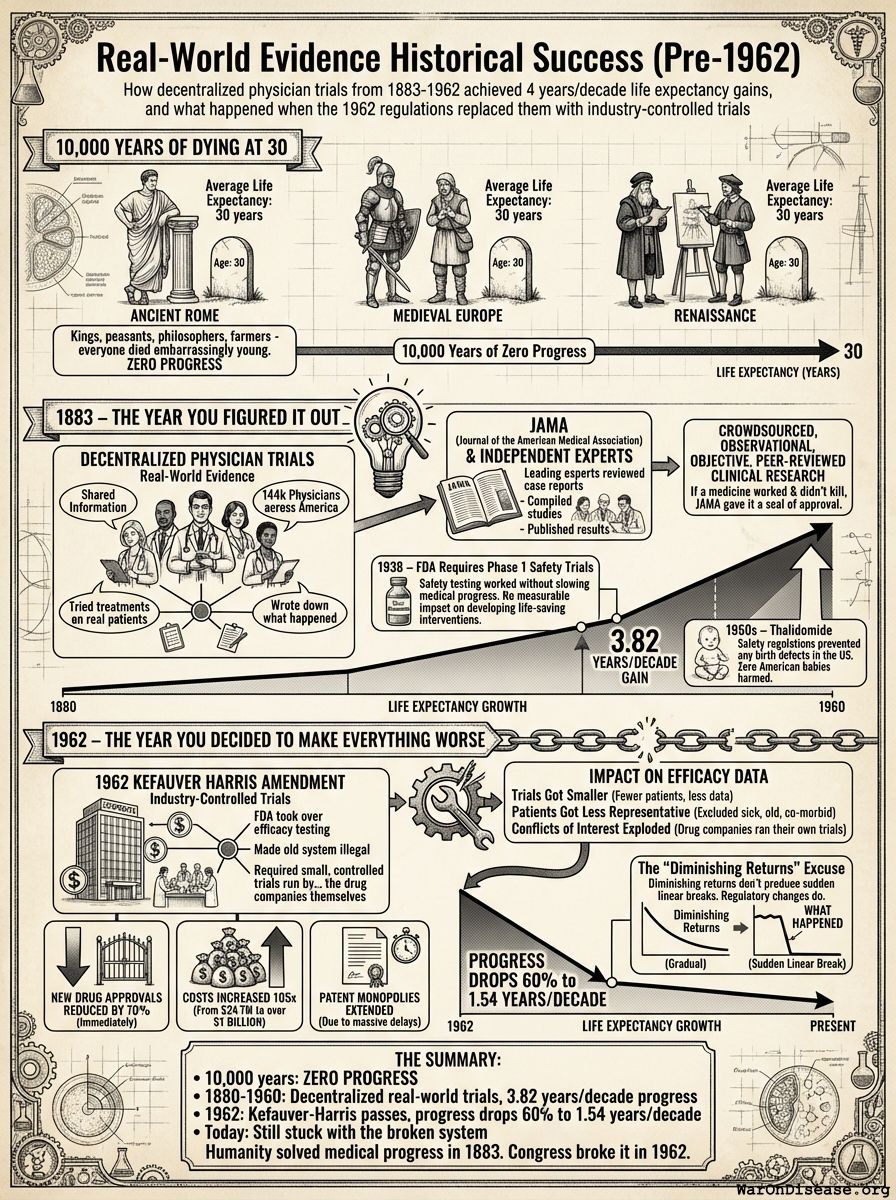
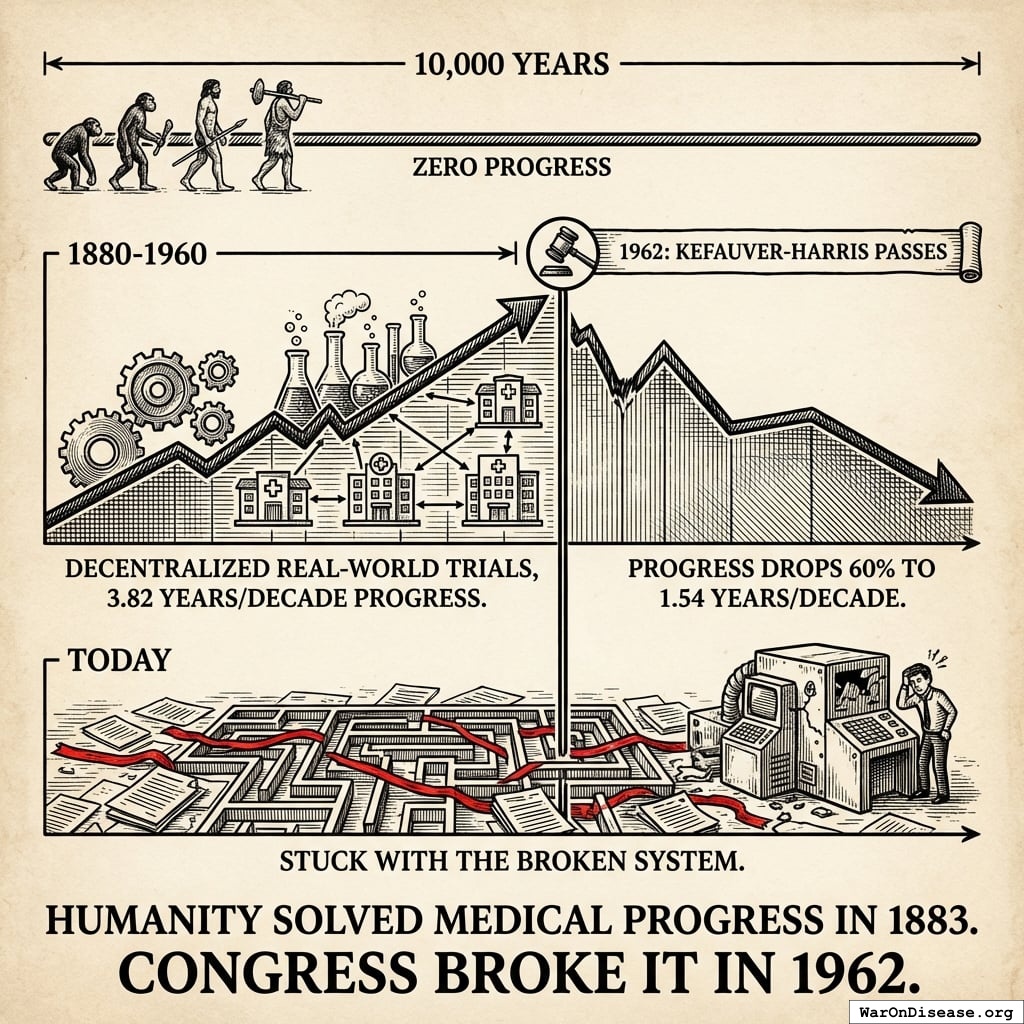
10,000 Years of Dying at 30
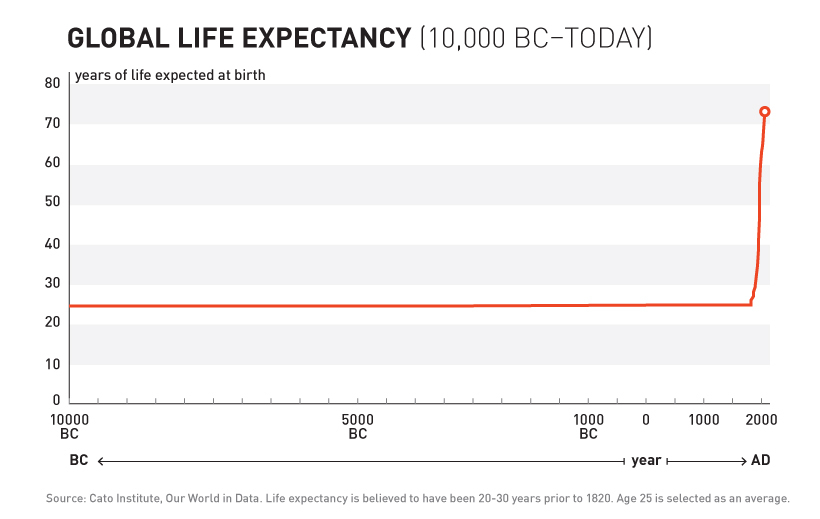
For over 99% of recorded human history, average life expectancy was 30 years138.
Ancient Rome: 30 years. Medieval Europe: 30 years. Renaissance: Still 30 years.
Kings, peasants, philosophers, farmers - everyone died embarrassingly young.
10,000 years. Zero progress.
1883 – The Year You Figured It Out
Then something changed.
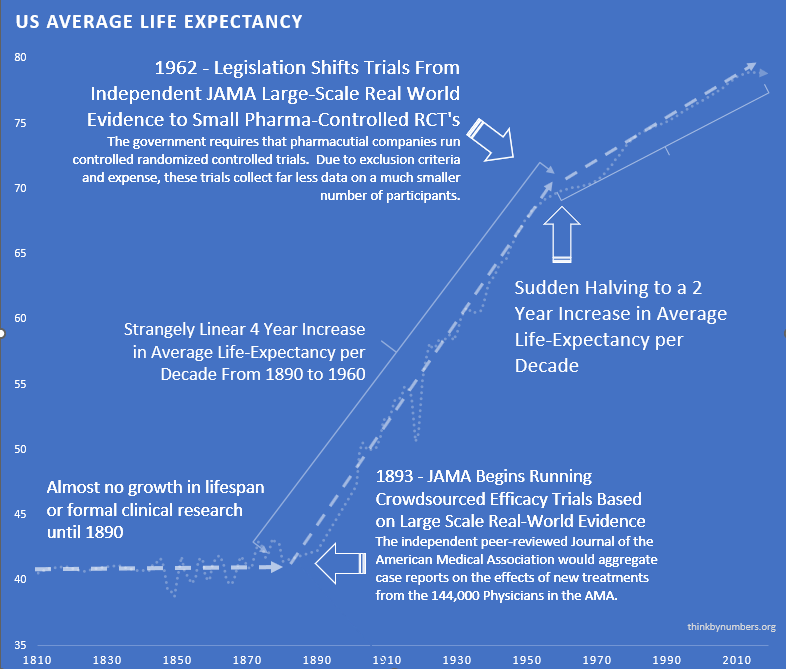
In 1883, doctors founded the Journal of the American Medical Association (JAMA)139 and did something novel: they shared information. After 10,000 years someone thought “what if we told each other what works?”
144k physicians physicians across America (see reference95) tried treatments on real patients. They wrote down what happened. “This drug helped.” “This drug killed the patient.” “This drug did nothing but made them smell funny.”
Leading experts reviewed these case reports, compiled them into studies, and published results. If a medicine worked and didn’t kill people, JAMA gave it a seal of approval.
Crowdsourced, observational, objective, peer-reviewed clinical research. And it worked.
The Result
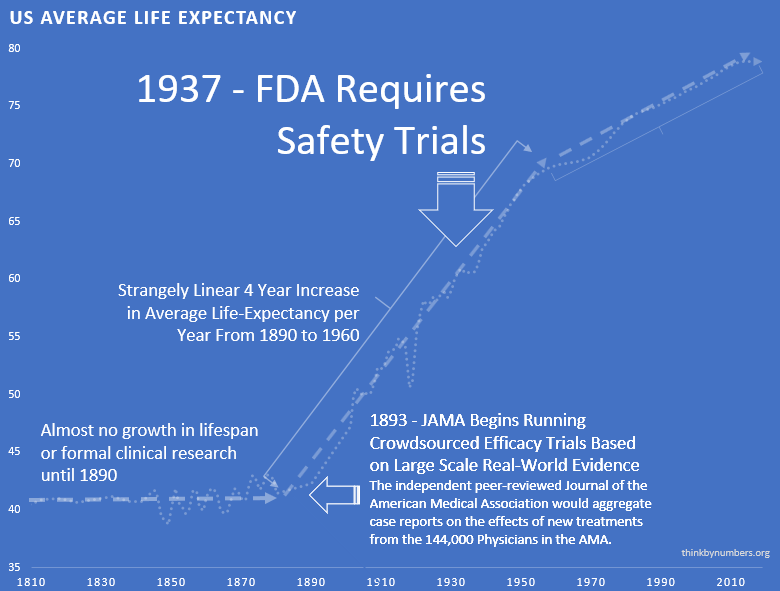
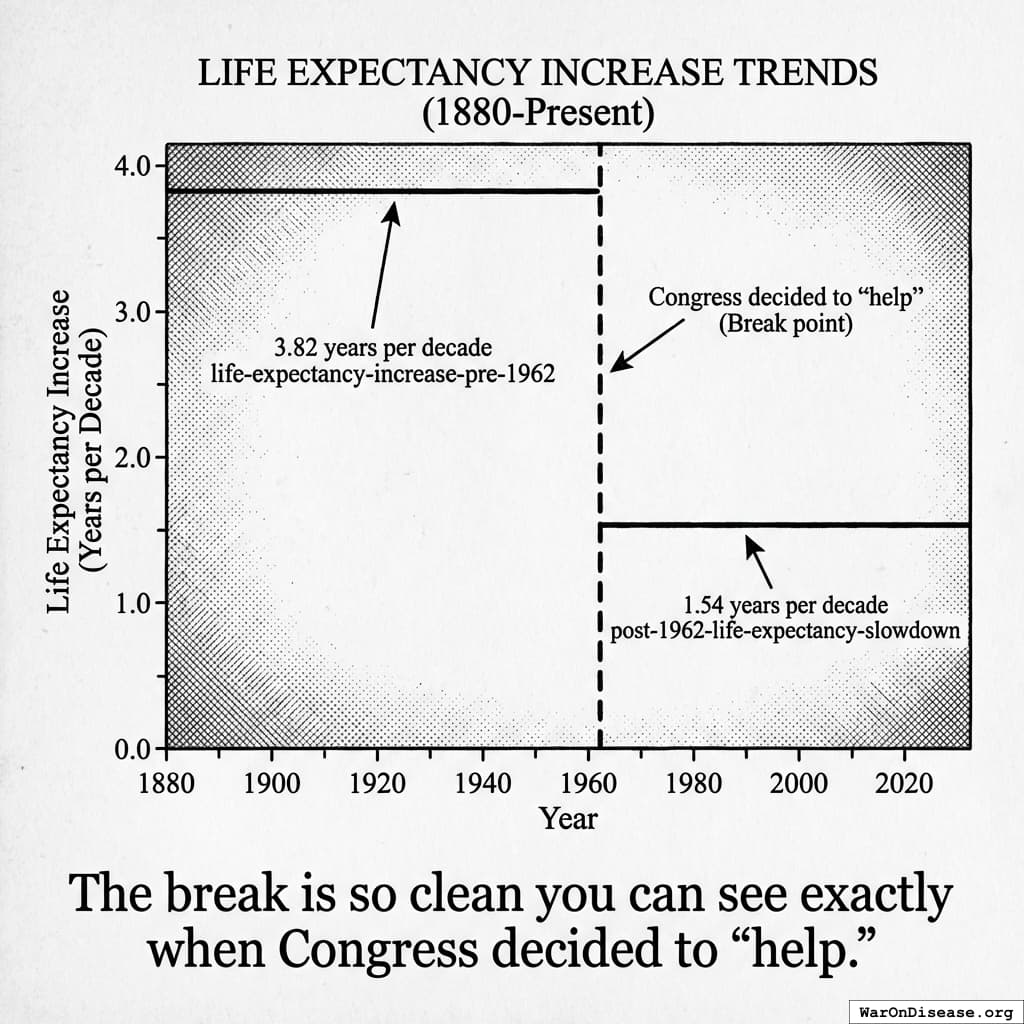
After 10,000 years of zero progress, human life expectancy suddenly increased by 3.82 years every decade123 (calculated from 1880-1960 data). For 80 years.
The most linear relationship in medical history. Suspiciously perfect. It lasted from 1883 to 1960.
1938 – The FDA Requires Phase 1 Safety Trials
Elixir sulfanilamide caused over 100 deaths in 1937.
Congress reacted by requiring all new drugs to include:
“adequate tests by all methods reasonably applicable to show whether or not such drug is safe for use under the conditions prescribed, recommended, or suggested in the proposed labeling thereof.”
These requirements evolved into the Phase 1 Safety Trial. This was reasonable - testing if a compound kills people before giving it to patients makes sense.
The consistent 3.82 years per decade increase123 in life expectancy remained unchanged before and after the new safety regulations. Safety testing worked without slowing medical progress.
The regulations had no measurable positive or negative impact on developing life-saving interventions.
1950s – Thalidomide: When Safety Regulations Actually Worked
Thalidomide was first marketed in Europe in 1957 for morning sickness. While it was initially thought to be safe in pregnancy, it resulted in thousands of horrific congenital disabilities140.
Existing FDA safety regulations prevented any birth defects in the US141. The 1938 safety testing framework worked exactly as intended. Zero American babies harmed.
Despite this success story for safety regulations, newspaper stories like the one below created public outcry for more regulation - targeting efficacy testing instead.
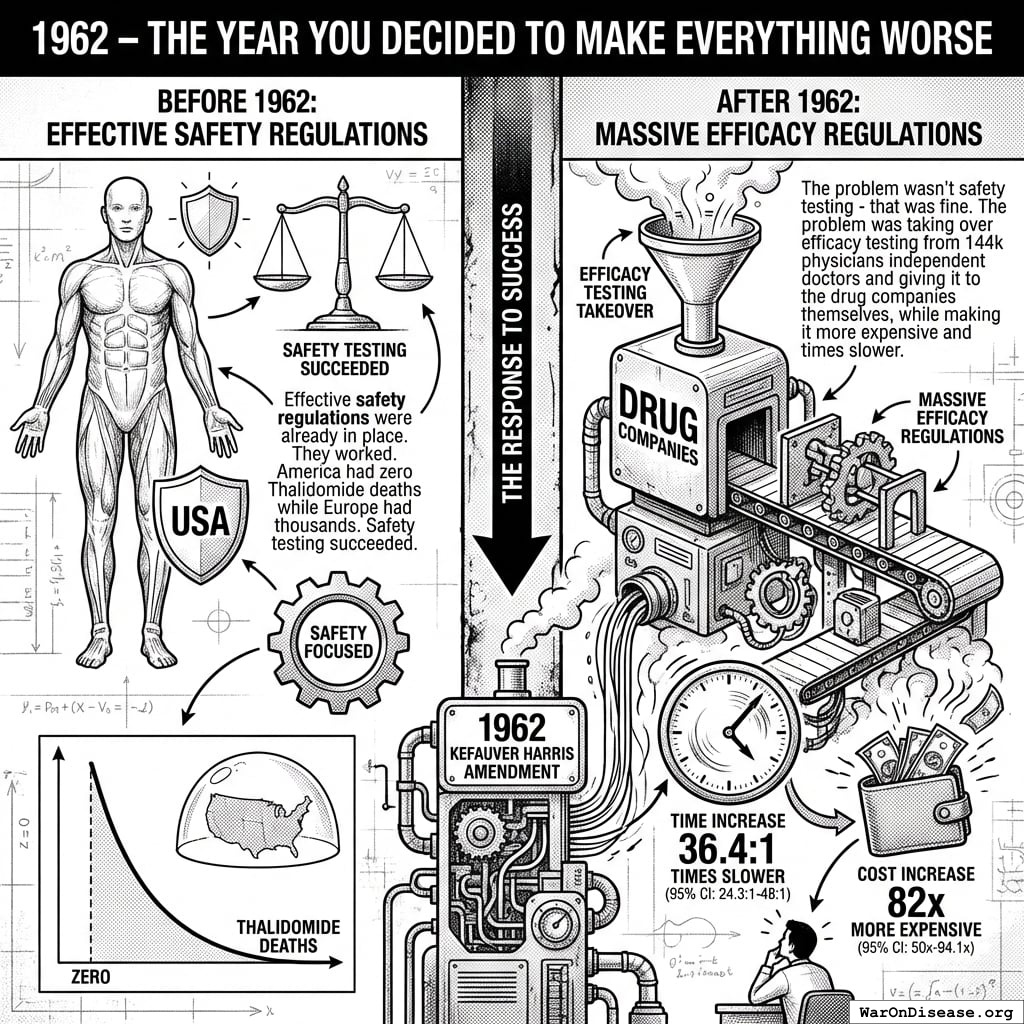
1962 – The Year You Decided to Make Everything Worse
Effective safety regulations were already in place. They worked. America had zero Thalidomide deaths while Europe had thousands. Safety testing succeeded.
So naturally, the government responded to this success by adding massive efficacy regulations via the 1962 Kefauver Harris Amendment142.
The problem wasn’t safety testing - that was fine. The problem was taking over efficacy testing from 144k physicians independent doctors and giving it to the drug companies themselves, while making it 82x (95% CI: 50x-94.1x) times more expensive and 42:1 (95% CI: 29.8:1-48:1) times slower.
What Changed
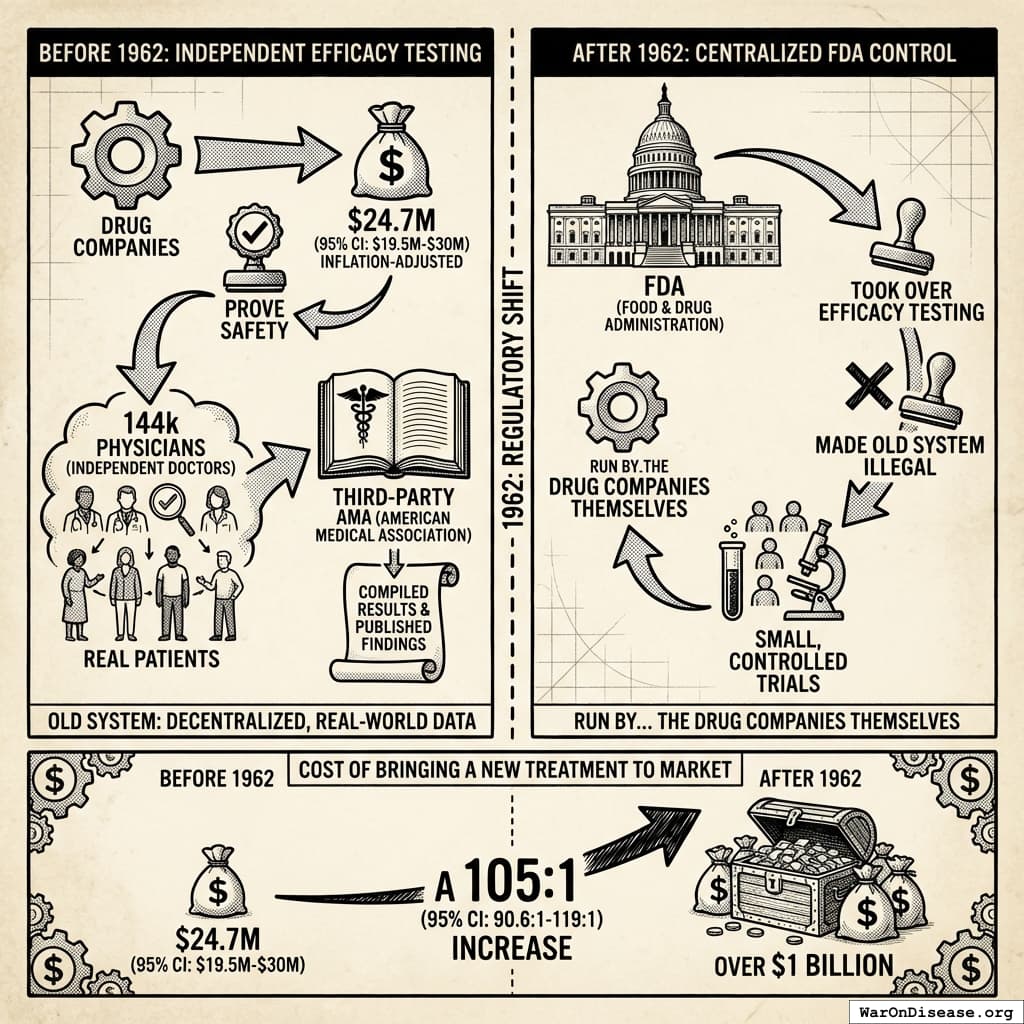
Before 1962:
- Drug companies spent $24.7M (95% CI: $19.5M-$30M) (inflation-adjusted) to prove safety (see reference33)
- Once approved as safe, 144k physicians independent doctors tested efficacy on real patients (see reference95)
- The third-party AMA compiled results and published findings
After 1962:
- FDA took over efficacy testing
- Made the old system illegal142
- Required small, controlled trials run by… the drug companies themselves
The irony: Regulations meant to ensure drugs work did this by:
- Banning large real-world trials
- Requiring small artificial trials
- Letting drug companies run their own tests
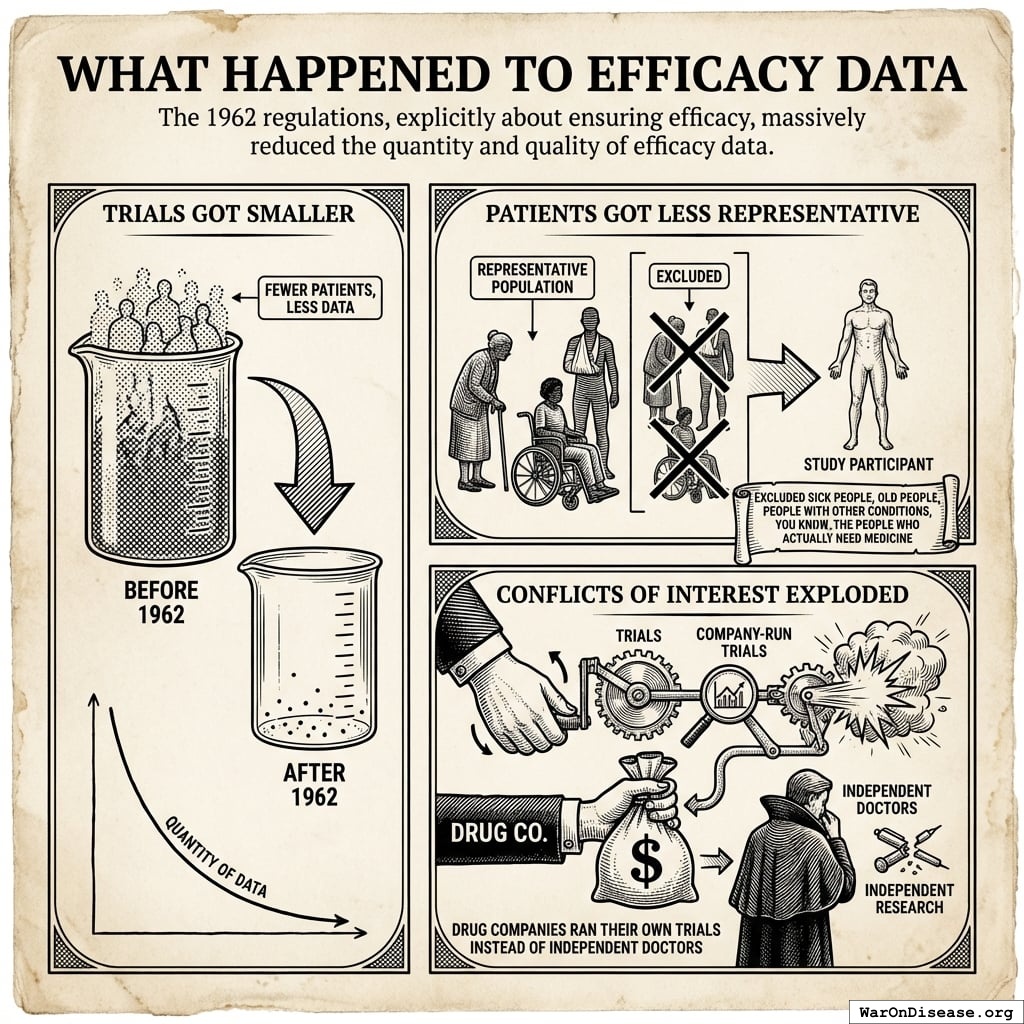
What Happened to Efficacy Data
The 1962 regulations, explicitly about ensuring efficacy, massively reduced the quantity and quality of efficacy data:
- Trials Got Smaller: Fewer patients, less data
- Patients Got Less Representative: Excluded sick people, old people, people with other conditions, you know, the people who actually need medicine
- Conflicts of Interest Exploded: Drug companies ran their own trials instead of independent doctors
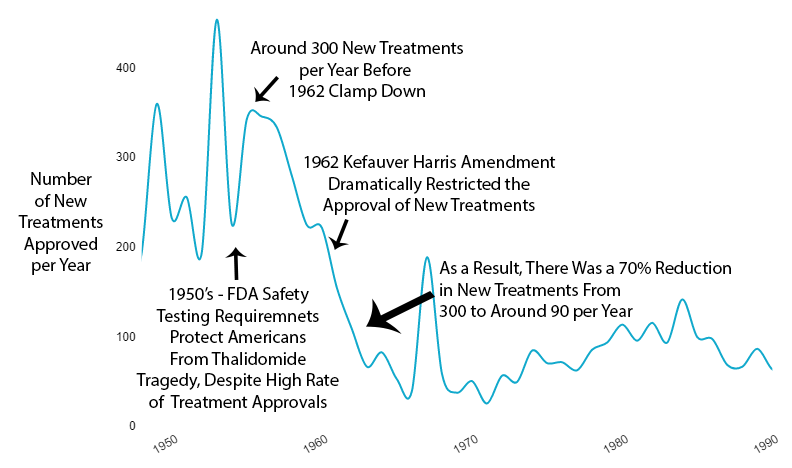
What Happened to New Treatments
The new regulations immediately reduced new drug approvals by 70% (see reference92).
Not gradually. Immediately.
What Happened to Costs
Since 1962, the cost of bringing a new treatment to market went from $24.7M (95% CI: $19.5M-$30M) to over $1 billion (source) (inflation-adjusted).
A 105x (95% CI: 90.6x-119x) increase. For worse results.
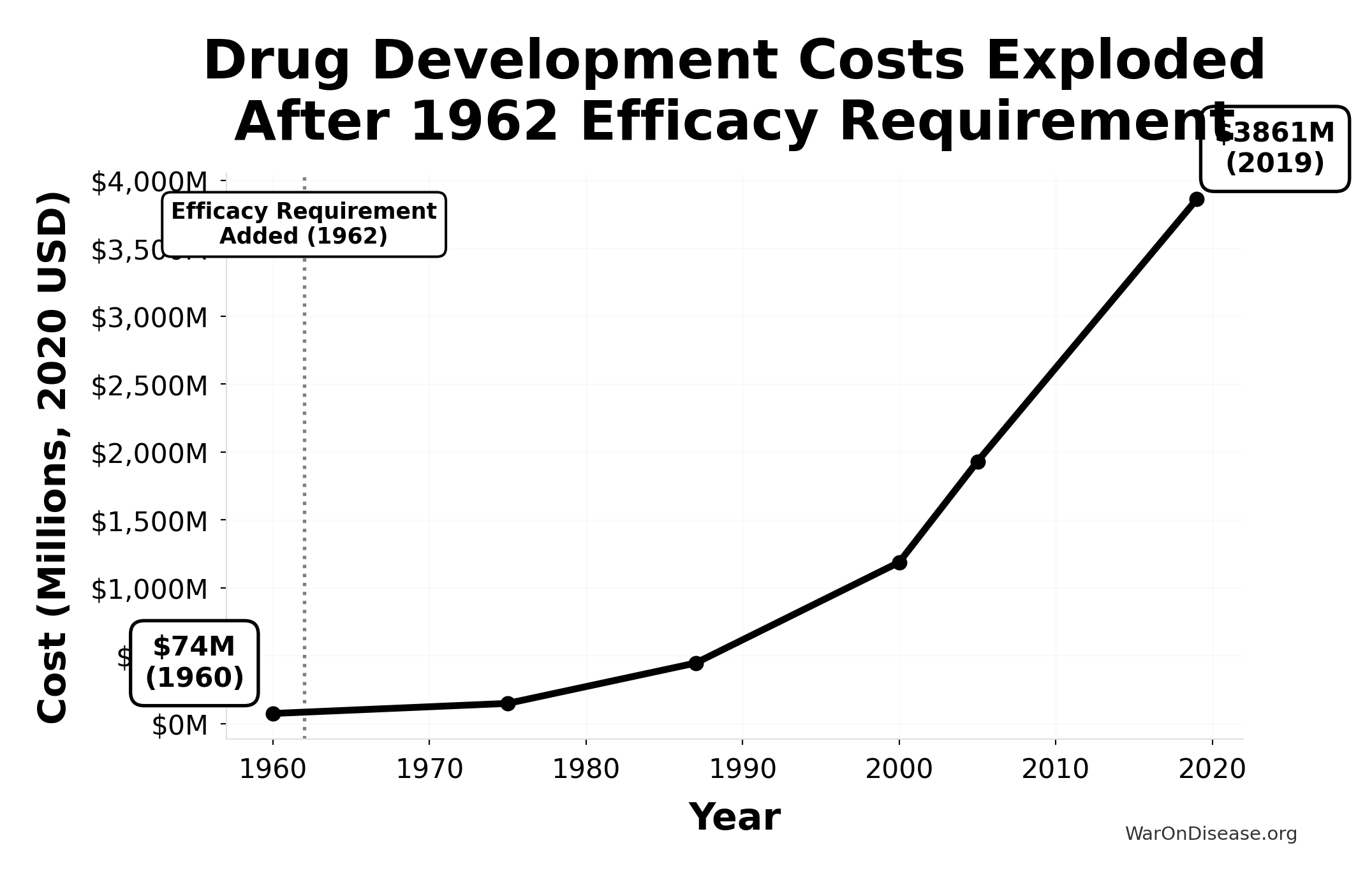
This chart shows the exponential increase in the cost to develop a single new drug from 1960-2019, adjusted for inflation to 2020 dollars. The vertical line marks the 1962 efficacy amendment (Kefauver-Harris Amendment).
Drug development costs were relatively stable before 1962, then exploded exponentially after the efficacy requirement was added. Costs increased 52x from $74M (1960) to $3,861M (2019), making innovation increasingly expensive and inaccessible.
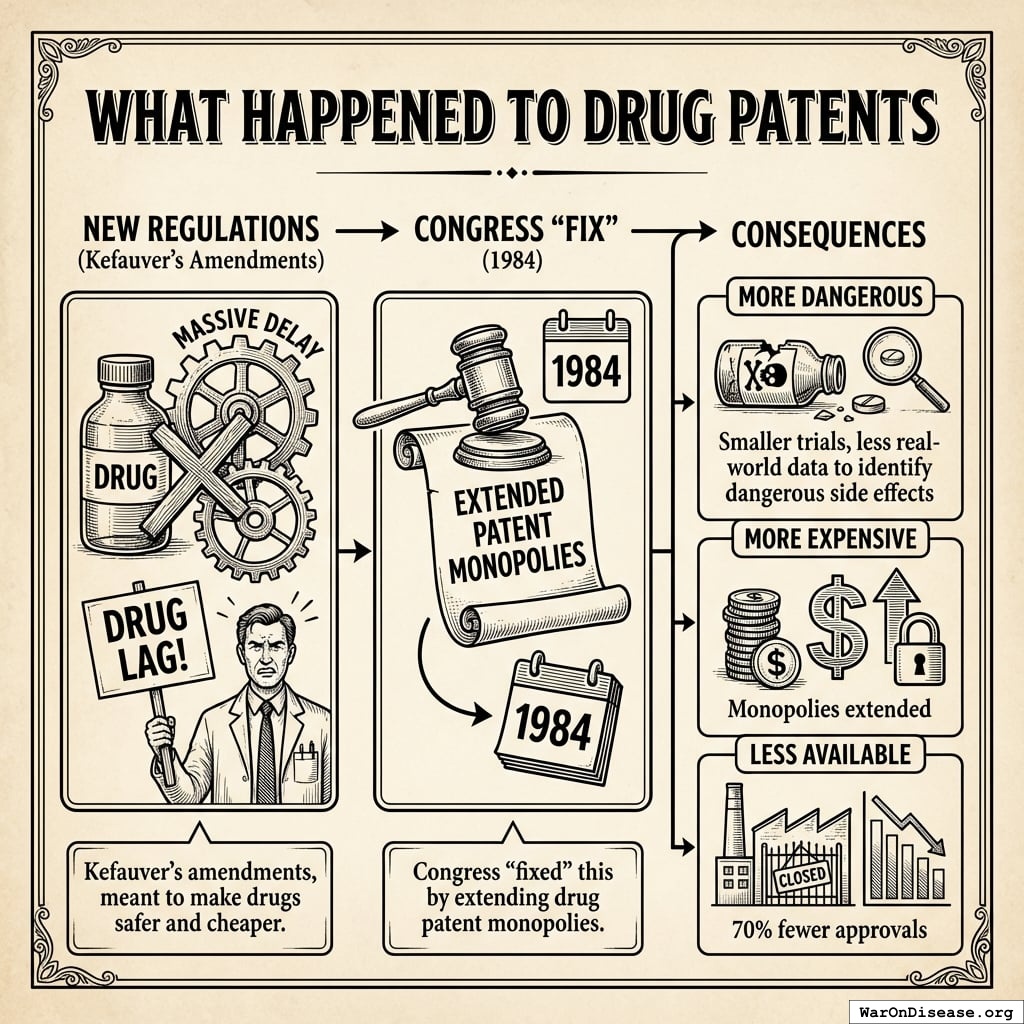
What Happened to Drug Patents
The new regulations created such massive delay that the pharmaceutical industry complained about a “drug lag.”
Congress “fixed” this by extending drug patent monopolies143 in 1984.
Kefauver’s amendments, meant to make drugs safer and cheaper, instead made them:
- More dangerous (smaller trials, less real-world data to identify dangerous side effects)
- More expensive (monopolies extended)
- Less available (70% fewer approvals)
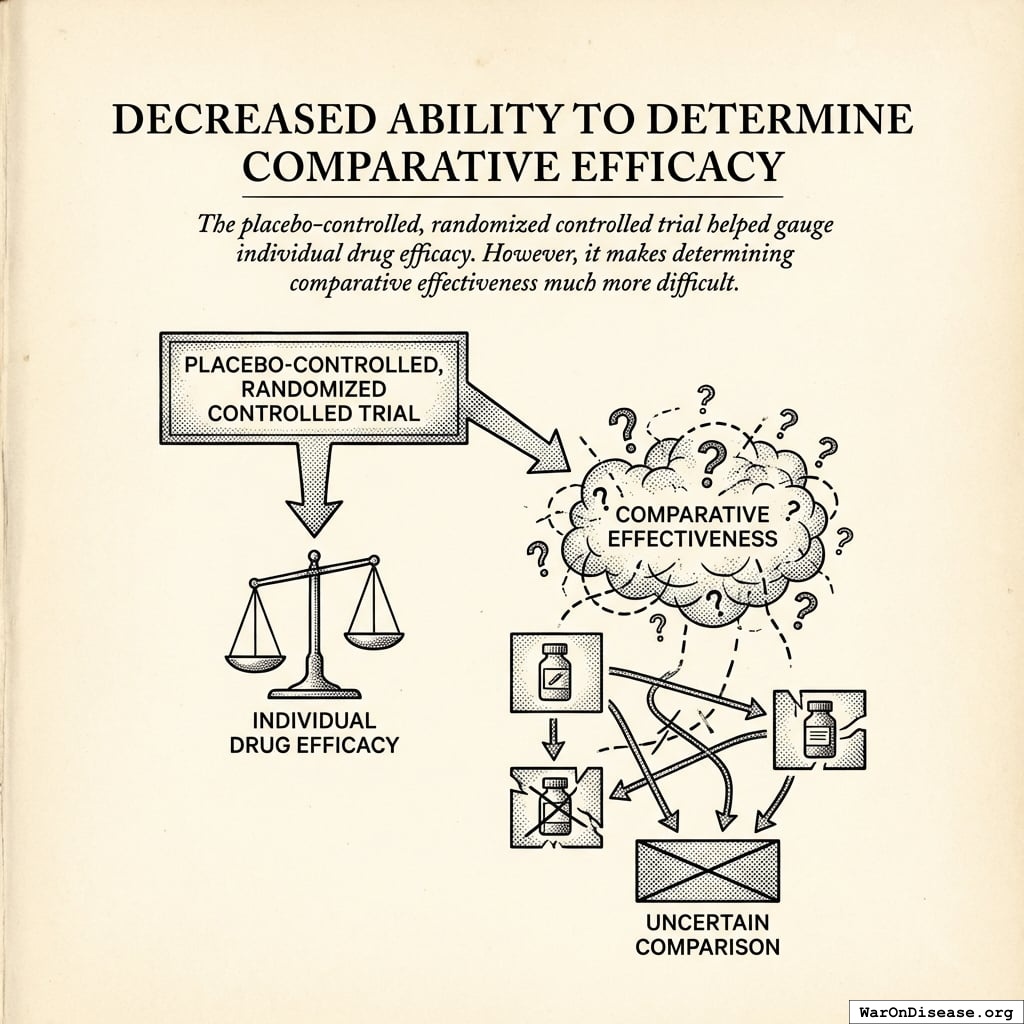
Decreased Ability to Determine Comparative Efficacy
The placebo-controlled, randomized controlled trial helped gauge individual drug efficacy. However, it makes determining comparative effectiveness much more difficult.
What Happened to Life Expectancy (The Part That Matters)
Remember that suspiciously linear 3.82-year increase123 in life expectancy every decade from 1883 to 1960?
Here’s what happened in 1962:
It strangely quickly dropped by 60%124. To 1.54 years per decade.
From 1880 to 1960: 3.82 years per decade123.
From 1962 onward: 1.54 years per decade124.
The break is so clean you can see exactly when Congress decided to “help.”
The “Diminishing Returns” Excuse
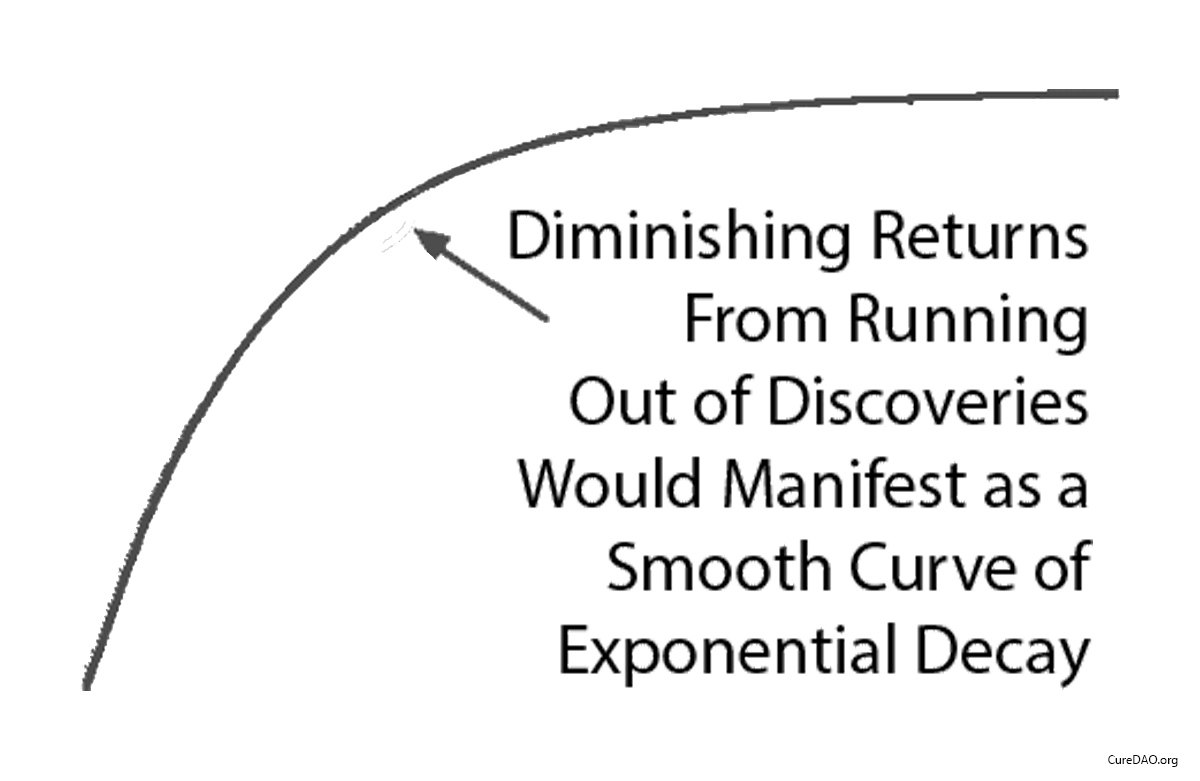
Some claim “diminishing returns explain the slowdown.”
Diminishing returns produce exponential decay. A smooth curve that gradually flattens over time.
This isn’t what happened.
What happened was a straight line that instantly changed slope in 1962 when the regulations changed.
Diminishing returns don’t produce sudden linear breaks. Regulatory changes do.
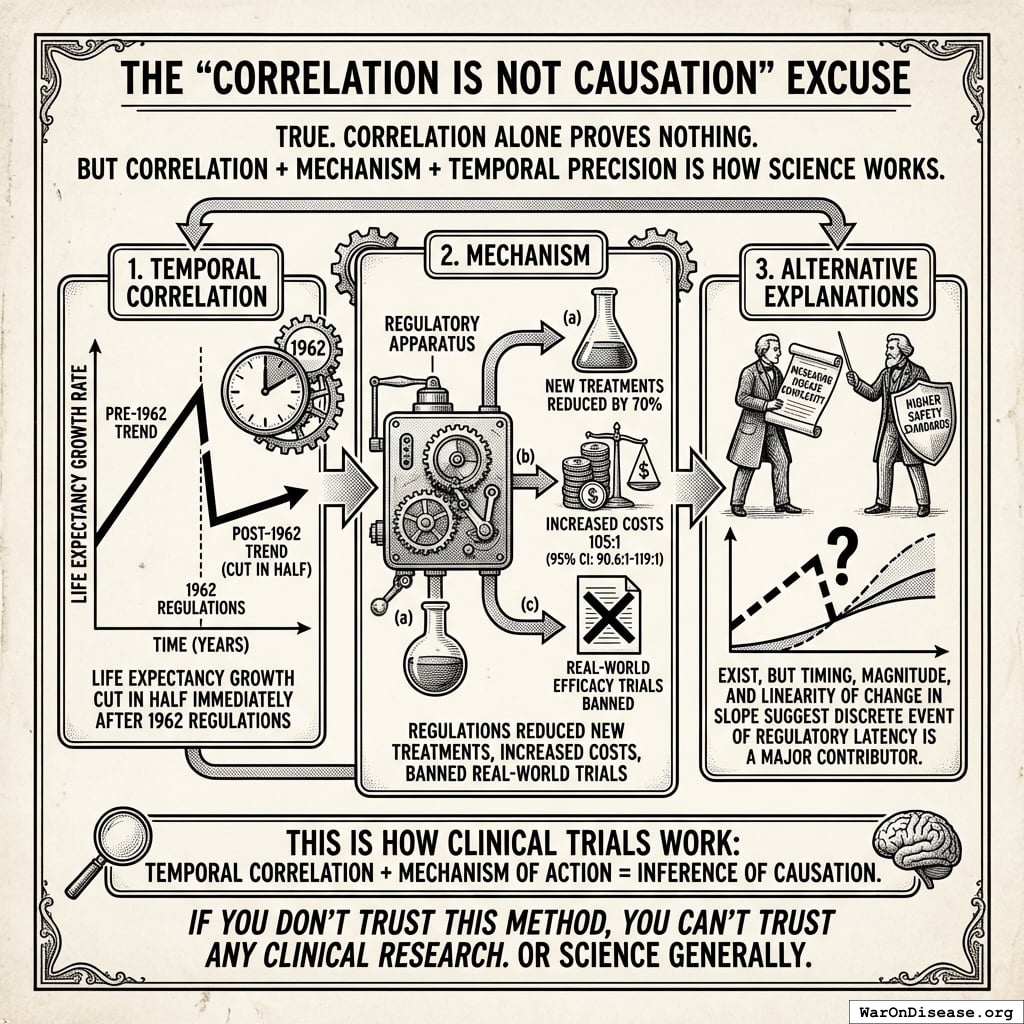
The “Correlation Is Not Causation” Excuse
True. Correlation alone proves nothing.
But correlation + mechanism + temporal precision is how science works.
Here’s what you have:
- Temporal correlation: Life expectancy growth cut in half immediately after 1962 regulations
- Mechanism: Regulations reduced new treatments by 70%, increased costs 105x (95% CI: 90.6x-119x), banned real-world efficacy trials
- Alternative explanations: Alternative explanations exist (increasing disease complexity, higher safety standards), but the timing and magnitude and linearity of the change in the slope after the break strongly suggest the discrete event of regulatory latency is a major contributor rather that other causes that would produce far more gradual change in the slope over time.
This is how clinical trials work: temporal correlation + mechanism of action = inference of causation.
If you don’t trust this method, you can’t trust any clinical research. Or science generally.
Impact of Innovative Medicines on Life Expectancy
A three-way fixed-effects analysis of 66 diseases in 27 countries suggests that if no new drugs had been launched after 1981, years of life lost would have been 2.16 times higher. It estimates pharmaceutical expenditure per life-year saved was $2,837.
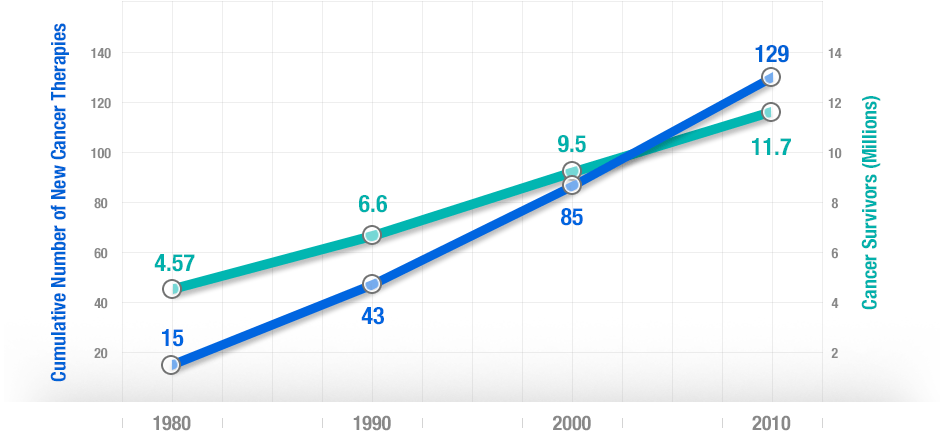
More people survive as more treatments are developed. There’s a strong correlation between developing new cancer treatments and cancer survival over 30 years.
The Summary
- 10,000 years: Zero progress
- 1880-1960: Decentralized real-world trials, 3.82 years/decade progress123
- 1962: Kefauver-Harris passes, progress drops 60%124 to 1.54 years/decade
- Today: Still stuck with the broken system
Humanity solved medical progress in 1883. Congress broke it in 1962.